Through drug efficacy tracking and screening, and components optimization, develop new drugs from TCM
active components
Total Alkaloid vesic gel of Semen Strychni
The effective components of total alkaloids (more than 80%) were isolated from Semen Strychni
The anti-inflammatory effect is better than that of the chemical drug Votalin.
Through New Preparation Vesicle Gel to reach sustained release, targeting, reducing toxicity and
improving effect
It’s a model of Toxic TCM innovation.
First clinical permission of TCM nano preparation.
Special support for major new drug development in Elevth Five Year Plan and Twelfth Five Year Plan.







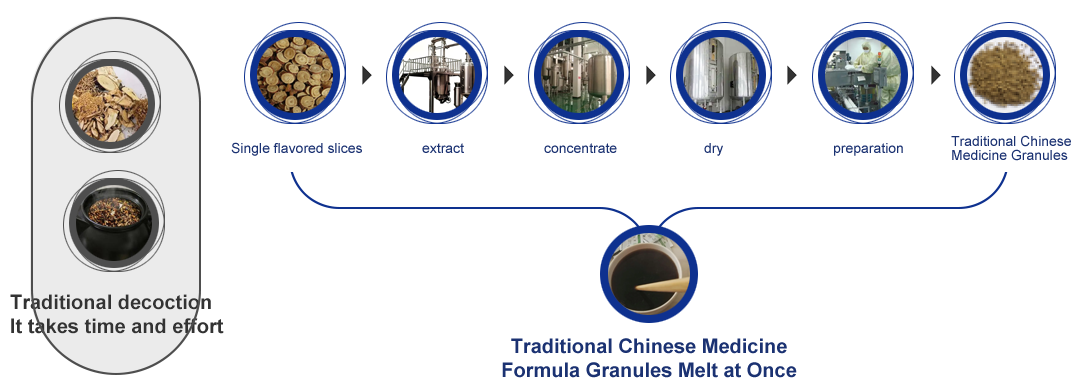
 TCM new drug process development technology system
TCM new drug process development technology system 